Abstract
Polymer-based dry and flexible electrodes are becoming increasingly popular in wearable healthcare for long-term electrocardiography (ECG) monitoring due to their comfort, biocompatibility, and gel-free operation. In this study, electrodes are prepared for ECG measurement using a polydimethylsiloxane (PDMS) and multi-walled conductive carbon nanotube (CNT)-based composite. The homogeneous dispersion of CNTs in PDMS is crucial for determining the electrical performance of the composite, and to accomplish this uniform dispersion, a combination of ultrasonication and a high-shear homogeneous dispersion technique is employed. This combination approach resulted in a rapid composite preparation method that took approximately 2–3 h to complete. The performance of the fabricated ECG electrodes was evaluated by measuring the ECG signal and compared to commercially available wet Ag/AgCl ECG electrodes. The ECG signal of the CNT/PDMS composite electrode with CNT content of 6 wt.% demonstrated optimal correlation of 0.9729 with the ECG signal measured from the Ag/AgCl electrode. The CNT/PDMS composite-based dry electrodes can be easily fabricated by manufacturing techniques, suggesting a possible benefit of cost and time reduction in mass production.
Similar content being viewed by others
Introduction
Continuous and ambulatory personal health monitoring is now possible with wearable health monitoring technologies, and rapid smartphone technology growth is predicted to enhance ubiquitous healthcare quality.1 Electrocardiography is an important bio-signal for the diagnosis of the cardiovascular system. Disposable Ag/AgCl hydrogel bioelectrodes or gel-based Ag/AgCl electrodes are widely used for electrocardiogram (ECG) monitoring.2 Although these wet electrodes have been widely used, they are unsuitable for long-term monitoring due to gel evaporation and skin irritation.3
Dry bioelectrodes work without an electrolyte layer between the skin and are a promising alternative to gel-based electrodes. Although many dry electrodes have been developed using metals, their practical use is limited due to poor biocompatibility, motion artefacts, and higher electrode–skin impedance.4 Soft polymer-based dry electrodes are adaptive to skin contours, biocompatible, comfortable, and suitable for the long-term acquisition of the ECG. Recent progress in soft and conductive polymer-based bioelectrodes has attracted considerable attention for biomedical applications such as soldier health monitoring, athlete performance monitoring, arrhythmia detection, heart rate variability analysis, and medication efficacy assessment.5,6,7
Polydimethylsiloxane (PDMS) is a widely used material as a stretchable polymer in biomedical applications due to its excellent biocompatibility, low cost, flexibility, low permeability, thermal stability, and mouldability.8 However, PDMS is an insulator with very low conductivity; this property of PDMS is a challenge for applications requiring a conductive soft conductive polymer. Additionally, connecting wires to PDMS is complicated as PDMS is not solderable. Attaching wires using electrically conductive adhesives or clam** contact pads is common to create an electrical path for an external circuit. Researchers have prepared conductive PDMS by blending various fillers for flexible electronics applications.9,10,11,12 Carbon nanotubes (CNTs) are a nanomaterial with high conductivity, high aspect ratio, good thermal conductivity, and excellent flexibility. Because of these properties, CNTs are effective filler materials for increasing the electrical/thermal conductivity of polymer or epoxy.13 Multi-walled carbon nanotubes (MWCNTs) are cost-effective compared to other CNT types, while maintaining a highly conductive property.
Research studies suggest that CNT/PDMS composite can effectively address challenges commonly associated with dry electrodes, such as high electrode-to-skin impedance, poor biocompatibility, and variations in contact area during motion. However, CNTs produced by mass production methods are entangled and tend to aggregate due to the strong van der Waals force among them. Therefore, the homogeneous dispersion of CNTs in a viscoelastic polymer solution is challenging. Researchers have tried various methods, such as simple stirring, three-roll milling, dispersion in liquids, and ultrasonication for the dispersion of CNTs in the polymer.14,15,16,17,18 However, the effectiveness of these methods varies due to the processing method and characteristics of CNTs, such as their aspect ratio, purity, dimension, and functionalization.19,20,21 CNT/PDMS can be integrated into field-effect transistor (FET) structures as sensing elements for the fabrication of flexible FET-based biosensors.22,23,24,25,26,27,28,29 This study explores a combination of high-shear mixing and ultrasonication procedures to prepare dry bioelectrodes for ECG monitoring out of CNT/PDMS composite material. Different CNT concentrations are explored for the fabrication of CNT/PDMS composite electrodes, and the impact of the CNT concentration on the electrode's performance is assessed.
Materials and Methods
Materials
Multi-walled carbon nanotubes with a purity level exceeding 95%, a length of 50 μm, an outer diameter ranging from 8 nm to 15 nm, and an inner diameter between 3 nm and 5 nm were obtained from XFNANO Materials Tech Co., Ltd., located in Nan**g, China. The PDMS polymer used in the experiment was acquired from Dow Corning as a two-part heat-curable silicone elastomer kit known as Sylgard® 184. Isopropyl alcohol (IPA) with a purity exceeding 99.9% was purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA).
CNT Composite
All techniques for preparing CNT-based composites must address the critical challenges of using CNT as nanofillers: poor dispersibility and a tendency to form bundles. Van der Waals forces lead to significant entanglement of CNTs, and when they form large bundles or dense agglomerates, it can result in uncontrolled electronic modifications and inferior device performance.30 Consequently, achieving uniform distributions of CNT within the PDMS polymer matrix becomes imperative to attain superior electrical performance in CNT/PDMS devices. One approach to mitigate these issues is functionalizing the surface of CNTs, which can weaken the attractive forces between nanotubes and reduce bundle formation. However, this method often results in CNT products with diminished characteristics.31
Solution mixing is the most widely used and straightforward method for creating composites. This technique involves introducing the nanofiller and polymeric matrix into a suitable solvent. Once the mixture achieves homogeneity, the solvent is evaporated, resulting in the formation of the composite.32 However, the polymers' solubility limits the solution mixing method's effectiveness. One can utilize melt processing as a viable technique for thermally durable polymers like polypropylene, polycarbonates, and similar materials to address the limitation of solution mixing. During melt processing, the polymer is combined with CNTs while molten. The main drawback of this technique is that the high viscosity of melted polymers makes it challenging to achieve a uniform distribution of CNTs within the matrix.33 In situ polymerization is a different strategy involving dispersing CNTs into the monomer matrix before starting the polymerization process after evenly distributing them throughout the system. In situ polymerization also has the advantage of using polymers that cannot be mixed in a solution or a melt mixing.34
Achieving uniform dispersion of CNT in polydimethylsiloxane is challenging because of the high viscosity of the PDMS monomer. Additionally, CNTs mass-manufactured with chemical vapour deposition are highly entangled and bundled (see Fig. 3a). CNTs must be dispersed in a low-viscosity solvent to address this issue before being mixed with the PDMS monomer.
This study addresses the challenges of achieving a uniform dispersion of carbon nanotubes in a polymer matrix by combining ultrasonication and high-shear homogenization techniques. Ultrasonication employs high-frequency sound waves to create cavitation bubbles, inducing mechanical agitation.35 This breaks down CNT agglomerates and disperses individual or small bundles of nanotubes. High-shear homogenization applies mechanical shear forces to further disperse and align CNTs,36 disrupting any remaining agglomerates not fully broken down by ultrasonication. This approach ensures thorough and uniform dispersion, preventing the formation of large clusters and promoting consistent mixing throughout the entire volume of the mixture.
Composite Preparation Process
The dispersion process in this study entails three crucial steps to effectively disperse carbon nanotubes inside a polymer matrix. The detailed procedure is illustrated in Fig. 1. The first step involves the dispersion of CNTs in a solvent through ultrasonication. The CNTs are initially mixed with a solvent—in this case, isopropyl alcohol (IPA)—using a probe sonicator. The probe sonicator is operated at a frequency of 20 kHz with power of 700 W. This ultrasonication step aims to break down agglomeration or bundles of entangled CNT and disperse them uniformly throughout the solvent. To preserve the structural integrity and aspect ratios of CNTs, it is necessary to limit the sonification time to 30 min.
Next, the PDMS monomer, which is the base material for the polymer matrix, is combined with the CNT suspension. The mixture is then subjected to further dispersion using a high-shear homogenizer. Specifically, a stator/rotor-based high-shear homogenizer, the T-18 ULTRA-TURRAX®, is employed. This homogenizer is operated at 10,000 rpm for 15 min. Using both high-shear homogenization and ultrasonication helps to achieve a homogeneous dispersion of the CNTs within the PDMS monomer, improving their uniform distribution throughout the substance. The vacuum filtration technique is applied to extract most of the IPA solvent. The mixture is then passed through a filter, facilitating the separation of the solvent from the CNT/PDMS solution. This crucial step helps to eliminate any excess solvent and prepares the mixture for subsequent processing. Afterwards, any remaining solvent is evaporated by placing the filtered mixture into a vacuum oven set at 85°C for 1 h. This heat treatment in the vacuum oven removes any residual traces of solvent, leaving behind a concentrated CNT/PDMS mixture.
Lastly, the curing agent is introduced to initiate the polymerization process. The curing agent is mixed with the CNT/PDMS mixture in a ratio of 10:1. The addition of the curing agent triggers a chemical reaction, leading to the cross-linking and hardening of the PDMS, forming a solid polymer composite. This final step ensures the stabilization and consolidation of the dispersed CNTs within the PDMS matrix. The mixture becomes very viscous for CNT content > 8 wt.%, making the fabrication of the composite difficult.
Fabrication of the CNT/PDMS Composite Electrode
The process for fabricating the CNT/PDMS electrode is depicted in Fig. 2 and involves several steps. First, a disposable ECG electrode is affixed to the central area of a 4-inch petri dish. This disposable electrode creates a negative mould for the subsequent steps. Next, the monomer of the PDMS kit and the curing agent are mixed in a 10:1 ratio. This mixture is then subjected to a vacuum to eliminate any trapped air bubbles. The PDMS mixture is transferred into the petri dish, covering the attached disposable electrode, and cured at room temperature for 48 h. After the curing process, the PDMS material is delicately separated from the petri dish and the disposable electrode, revealing the negative PDMS mould. A snap connector, a crucial component for the electrode's functionality, is carefully positioned inside the negative PDMS mould. The prepared CNT/PDMS dispersion is poured into the mould, ensuring the entire cavity is filled. Subsequently, the mould containing the CNT/PDMS dispersion is subjected to a vacuum to remove any remaining air bubbles that may have formed during the pouring process. The composite electrode is then cured at 110°C for 1 h. Finally, the cured CNT/PDMS composite electrode is meticulously removed from the mould.
Results and Discussion
Dispersion Characterization
The structure of the CNT/PDMS composite (concentration: 8 wt.%) was characterized using Fourier transform infrared (FTIR) spectroscopy and field emission scanning electron microscopy (FE-SEM). Figure 3b presents the cross-sectional views of the CNT/PDMS film captured by SEM. These images reveal the absence of any loose CNTs on the composite's surface, indicating their complete penetration into the film and the formation of a percolation network. This phenomenon contributes to enhanced electrical and mechanical properties of the material.
The FTIR spectra of the CNT/PDMS composite were obtained using an FTIR spectrometer (IRTracer-100, Shimadzu Corporation, Kyoto, Japan) within the range of 500–4000 cm−1. Figure 4 illustrates the identified and labelled unique peaks in the FTIR spectra of the composite material.
The absorption band at 2960 cm−1 indicates the C–H stretching of CH3, while the peak at 1407 cm−1 represents the C=C bending vibration of carbon nanotubes.37 The presence of the polysiloxane group in PDMS was confirmed by observing the Si–CH3 bands at 1243 cm−1 and in the 680–862-cm−1 regions.38 Furthermore, the wide absorption band at 1009 cm−1 corresponds to the symmetrical stretching of Si–O–Si, which indicates the successful synthesis of the CNT/PDMS composite.39
Electrical Properties
Before the percolation threshold was reached, the CNTs inside the PDMS matrix were distributed randomly, leading to isolated fillers and no percolation networks; consequently, the electron movement mechanism involved hop** along adjacent CNTs. However, once the concentration of CNTs exceeded the percolation threshold, a network was established throughout the system that allowed electrons to move along the conductive networks.40 Crossing the percolation threshold resulted in a metal-like conduction behaviour in the composites. The conductivity of CNT/PDMS was measured with Fluke PM6306 meter (see Fig. 5a). The electrical conductivity of CNT/PDMS composites with CNT concentrations of 2 wt.%, 4 wt.%, 6 wt.%, 8 wt.%, and 9 wt.% in the PDMS polymer matrix is illustrated in Fig. 5b. As the amount of CNT in the PDMS was increased, the composite conductivity also increased.
Mechanical Properties
The mechanical hardness of the CNT/PDMS composite was tested with an ASTM D 2240-compliant Shore A durometer (see Fig. 6a) and was found to increase with an increase in CNT content. Figure 6b illustrates this relationship between hardness and CNT content. The softness of polymer is crucial factor for development of dry ECG electrodes.41 The challenges associated with a rigid polymer include high motion artefacts, lower user comfort, complications in the polymer de-moulding process, and the formation of brittle electrodes. When considering the application of CNT/PDMS for dry ECG electrodes, it is crucial to have a balance between high electrical conductivity and low mechanical hardness. Notably, a composition with 2 wt.% CNT is not suitable due to its low conductivity, while a 9 wt.% composition is not suitable due to its elevated mechanical hardness.
Comparison of Time Required to Prepare CNT/PDMS Composite
The preparation time for CNT/PDMS composites can be relatively long due to several factors. First, the dispersion of CNTs within the PDMS matrix requires careful attention to achieve a uniform distribution. This requirement of uniform distribution is often addressed by sonication and mixing techniques, which can be time-consuming. Additionally, the CNT may require purification and functionalization processes before incorporation into the PDMS, further adding to the preparation time. The combination of high-shear homogenization and ultrasonication proposed in this study significantly reduce preparation time. Table I compares the composite preparation time of published studies with the current study.
Evaluation of CNT/PDMS Electrode for ECG Measurement
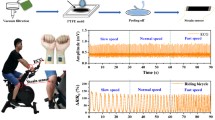
Conductive polymer electrodes with optimal CNT concentrations ranging from 4 wt.% to 8 wt.% were utilized for ECG recordings. The dry electrode was secured to the skin using micropore paper tape, and the recording system was connected to the dry electrode through a snap connector, as depicted in Fig. 7b. For comparison between the dry and wet electrodes, conventional wet electrodes were positioned close to the dry electrodes to acquire similar ECG signals (see Fig. 7b). A total of two pairs of wet-dry electrodes were placed on the subject's left and right arm, with one wet electrode designated as the ground electrode in lead-I configuration. The BIOPAC MP-150 data acquisition system was used for ECG acquisition, as shown in Fig. 6a. This system has a 150 Hz low-pass filter (LPF), a 0.01 Hz high-pass filter, and a 50 Hz notch filter.
The ECG signals obtained with the CNT/PDMS electrodes were comparable to those obtained with the Ag/AgCl electrodes, and no significant differences in the results were observed for the ECG features such as the P wave, QRS complex, and T wave (see Fig. 8).
The study also examined how different concentrations of CNTs affected the amplitude of the ECG signal. In Fig. 9, the peak-to-peak amplitude of the ECG electrodes made from the composite material is compared to that of Ag/AgCl wet electrodes using a boxplot. The ECG signal from electrodes with filler concentrations of 4 wt.%, 6 wt.%, and 8 wt.% showed a correlation of 0.9574, 0.9729, and 0.9793, respectively, with the ECG signal from Ag/AgCl wet electrodes. The correlation was calculated using Pearson's correlation coefficient.42 The ECG signal from the composite electrode containing 8 wt.% CNT content exhibits the highest correlation of 0.9793 with the ECG signal recorded from the Ag/AgCl electrode. However, this correlation is not significantly improved compared to the correlation of 0.9729 observed for the 6 wt.% concentration. Therefore, based on these results, it is recommended to use a concentration of 6 wt.% for fabricating ECG electrodes.
Long-Term ECG Measurement
This study evaluated the CNT/PDMS composite-based electrode for long-term use by recording ECG signals at 24-h intervals over 4 days after attaching the electrodes. The comparison was made between ECG signals acquired from CNT/PDMS composite-based electrode and Ag/AgCl electrode. To ensure consistent comparisons, a new Ag/AgCl electrode was applied to the skin as a reference electrode for each ECG recording session, as shown in Fig. 10.
Figure 11 shows the comparison of simultaneously acquired ECG signals from the reference Ag/AgCl electrode, CNT/PDMS composite-based electrode, and Ag/AgCl electrode. Average peak-to-peak amplitudes and ECG signal correlations between the reference Ag/AgCl electrode and electrodes under test are given in Table II. The results indicated that the Ag/AgCl electrode experienced a noticeable decrease in ECG signal amplitude and correlation over time due to the conductive gel drying out. However, the CNT/PDMS electrode showed no significant changes in ECG signals. This demonstrates that the CNT/PDMS electrode maintains its performance over a long period of time and does not degrade, making it an excellent choice for continuous ECG monitoring.
Skin Compatibility Test
The skin compatibility test was conducted by affixing both the Ag/AgCl disposable electrode and the CNT/PDMS composite-based electrode to the forearm using a microporous adhesive bandage for 4 days. The outcomes of the skin reaction are illustrated in Fig. 12. Prolonged usage of the wet electrode led to undesirable effects like skin swelling and redness, while the skin exhibited normal conditions and showed relatively low signs of irritation such as itching and redness when the nanocomposite sheet was employed. These findings confirm the excellent compatibility of the CNT/PDMS electrode with the skin.
Conclusion and Future Work
This study concludes that when CNTs as a filler are homogeneously incorporated into a PDMS polymer matrix by combining ultrasonication and high-shear homogenization dispersion techniques, this combination methods results in a composite preparation method that takes approximately 2–3 h to complete. The FTIR and FE-SEM characterization confirmed the homogeneous dispersion of the CNTs in the PDMS polymer matrix. ECG electrodes were fabricated from prepared CNT/PDMS composite, and the properties of CNT/PDMS composite polymer electrodes were evaluated in terms of conductivity, hardness, and ECG measurements. Based on the results, it can be concluded that increasing the CNT content in the composite electrode to 8 wt.% does not yield a significant improvement in the correlation with the ECG signal obtained from the Ag/AgCl electrode compared to the 6 wt.% concentration. Hence, a 6 wt.% concentration of CNT/PDMS composite is recommended for fabrication of dry ECG electrodes. The CNT/PDMS electrodes exhibited overall excellent performance and reliability in terms of signal quality, durability, and skin compatibility when compared to conventional wet electrodes. These findings support the feasibility and effectiveness of CNT/PDMS electrodes for ECG measurements, making them a promising alternative in medical applications.
Further optimization investigations can be carried out to determine the concentration of carbon nanotubes that achieves an optimal balance among electrical conductivity, flexibility, and biocompatibility. Additionally, the exploration of surface modification for CNTs shows promise, offering the potential to improve their dispersion within the PDMS matrix. The functionalization of CNTs with appropriate surface groups or coatings could result in a more even dispersion, ultimately enhancing electrical performance. Lastly, the incorporation of other nanomaterials alongside CNTs is worth exploring, capitalizing on the multifunctional properties of various nanoparticles to synergistically improve the overall performance of the composite material.
Data Availability
The authors confirm that the data presented in this manuscript are accurate and transparent. Original data are available upon request for validation.
References
M. Saleemi, M. Anjum, and M. Rehman, Ubiquitous healthcare: a systematic map** study. J. Ambient. Intell. Humaniz. Comput. 14, 5021 (2023).
J. Webster, Medical Instrumentation: Application and Design, 4th ed., (New York: Wiley, 2009).
G. Li, S. Wang, and Y.Y. Duan, Towards gel-free electrodes: a systematic study of electrode-skin impedance. Sens. Actuators B Chem. 241, 1244 (2017).
N. Meziane, J.G. Webster, M. Attari, and A.J. Nimunkar, Dry electrodes for electrocardiography. Physiol. Meas. 34, 47 (2013).
H.C. Jung, J.H. Moon, D.H. Baek, J.H. Lee, Y.Y. Choi, J.S. Hong, and S.H. Lee, CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 59, 1472 (2012).
K.E. Friedl, Military applications of soldier physiological monitoring. J. Sci. Med. Sport 21, 1147 (2018).
H. Ali, H.H. Naing, and R. Yaqub, An Iot assisted real-time high CMRR wireless ambulatory ECG monitoring system with arrhythmia detection. Electronics (Switzerland) 10, 1871 (2021).
H. Cong and T. Pan, Photopatternable conductive PDMS materials for microfabrication. Adv. Funct. Mater. 18, 1912 (2008).
H.L. Peng, J.Q. Liu, H.C. Tian, B. Xu, Y.Z. Dong, B. Yang, X. Chen, and C.S. Yang, Flexible dry electrode based on carbon nanotube/polymer hybrid micropillars for biopotential recording. Sens. Actuators A Phys. 235, 48 (2015).
Y. Yamamoto, D. Yamamoto, M. Takada, H. Naito, T. Arie, S. Akita, and K. Takei, Efficient skin temperature sensor and stable gel-less sticky ECG sensor for a wearable flexible healthcare patch. Adv. Healthc. Mater. 6, 1700 (2017).
A.A. Chlaihawi, B.B. Narakathu, S. Emamian, B.J. Bazuin, and M.Z. Atashbar, Development of printed and flexible dry ECG electrodes. Sens. Biosens. Res. 20, 9 (2018).
M. Chi, J. Zhao, Y. Dong, and X. Wang, Flexible carbon nanotube-based polymer electrode for long-term electrocardiographic recording. Materials 12, 971 (2019).
G. Mittal, V. Dhand, K.Y. Rhee, S.J. Park, and W.R. Lee, A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 21, 11 (2015).
J.H. Lee, Y.W. Nam, H.C. Jung, D.H. Baek, S.H. Lee, and J.S. Hong, Shear induced CNT/PDMS conducting thin film for electrode cardiogram (ECG) electrode. Biochip. J. 6, 91 (2012).
B. Liu, Y. Chen, Z. Luo, W. Zhang, Q. Tu, and X. **, A novel method of fabricating carbon nanotubes-polydimethylsiloxane composite electrodes for electrocardiography. J. Biomater. Sci. Polym. Ed. 26, 1229 (2015).
J.H. Kim, J.Y. Hwang, H.R. Hwang, H.S. Kim, J.H. Lee, J.W. Seo, U.S. Shin, and S.H. Lee, Simple and cost-effective method of highly conductive and elastic carbon nanotube/polydimethylsiloxane composite for wearable electronics. Sci. Rep. 8, 1375 (2018).
J. Jung, S. Shin, and Y.T. Kim, Dry electrode made from carbon nanotubes for continuous recording of bio-signals. Microelectron. Eng. 203–204, 25 (2019).
S. Masihi, M. Panahi, D. Maddipatla, A.J. Hanson, S. Fenech, L. Bonek, N. Sapoznik, P.D. Fleming, B.J. Bazuin, and M.Z. Atashbar, Development of a flexible wireless ECG monitoring device with dry fabric electrodes for wearable applications. IEEE Sens. J. 22, 11223 (2022).
Y.Y. Huang and E.M. Terentjev, Tailoring the electrical properties of carbon nanotube-polymer composites. Adv. Funct. Mater. 20, 4062 (2010).
D.W. Johnson, B.P. Dobson, and K.S. Coleman, A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 20, 367 (2015).
J. Chen and L. Yan, Effect of carbon nanotube aspect ratio on the thermal and electrical properties of epoxy nanocomposites. Fuller. Nanotubes Carbon Nanostruct. 26, 697 (2018).
A. Das, S. Rewari, B.K. Kanaujia, S.S. Deswal, and R.S. Gupta, Numerical modeling of a dielectric modulated surrounding-triple-gate germanium-source MOSFET (DM-STGGS-MOSFET)-based biosensor. J. Comput. Electron. 22, 742 (2023).
A. Das, S. Rewari, B.K. Kanaujia, and R.S. Gupta, Recent technological advancement in surrounding gate MOSFET for biosensing applications–a synoptic study. Silicon 14, 5133 (2022).
A. Das, S. Rewari, B.K. Kanaujia, S.S. Deswal, and R.S. Gupta, Ge/Si interfaced label free nanowire BIOFET for biomolecules detection–analytical analysis. Microelectron. J. 138, 105832 (2023).
A. Das, S. Rewari, B.K. Kanaujia, S.S. Deswal, and R.S. Gupta, Analytical investigation of a triple surrounding gate germanium source metal–oxide–semiconductor field-effect transistor with step graded channel for biosensing applications. Int. J. Numer. Model. Electron. Netw. Devices Fields 36, e3106 (2023).
A. Das, S. Rewari, B.K. Kanaujia, S.S. Deswal, and R.S. Gupta, Analytical modeling and do** optimization for enhanced analog performance in a Ge/Si interfaced nanowire MOSFET. Phys. Scr. 98, 074005 (2023).
A. Das, S. Rewari, B.K. Kanaujia, S.S. Deswal, and R.S. Gupta, Physics based numerical model of a nanoscale dielectric modulated step graded germanium source biotube FET sensor: modelling and simulation. Phys. Scr. 98, 115013 (2023).
A. Das, B.K. Kanaujia, V. Nath, S. Rewari, and R.S. Gupta, Impact of reverse gate oxide stacking on gate all around tunnel FET for high frequency analog and RF applications. 2020 IEEE 17th India Council International Conference, INDICON 2020 (Institute of Electrical and Electronics Engineers Inc., 2020)
A. Das, B.K. Kanaujia, S.S. Deswal, S. Rewari, and R.S. Gupta, Do** induced threshold voltage and ION/IOFFratio modulation in surrounding gate MOSFET for analog applications. Proceedings of 2022 IEEE International Conference of Electron Devices Society Kolkata Chapter, EDKCON 2022 (Institute of Electrical and Electronics Engineers Inc., 2022), p. 75–80
S. Tamayo-vegas, A. Muhsan, C. Liu, M. Tarfaoui, and K. Lafdi, the effect of agglomeration on the electrical and mechanical properties of polymer matrix nanocomposites reinforced with carbon nanotubes. Polymers (Basel) 14, 1842 (2022).
T.L. do Amaral-Montanheiroa, F.H. Cristóvan, J.P.B. Machado, D.B. Tada, N. Durán, and A.P. Lemes, Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 760, 55 (2014).
Y. Zhao, E.D. Cabrera, D. Zhang, J. Sun, T. Kuang, W. Yang, M.J. Lertola, A. Benatar, J.M. Castro, and L.J. Lee, Ultrasonic processing of MWCNT nanopaper reinforced polymeric nanocomposites. Polymer (Guildf) 156, 85 (2018).
I. Alig, T. Skipa, D. Lellinger, and P. Pötschke, Destruction and formation of a carbon nanotube network in polymer melts: rheology and conductivity spectroscopy. Polymer (Guildf) 49, 3524 (2008).
B.G. Cho, S. Lee, S.H. Hwang, J.H. Han, H.G. Chae, and Y.B. Park, Influence of hybrid graphene oxide-carbon nanotube as a nano-filler on the interfacial interaction in nylon composites prepared by in situ interfacial polymerization. Carbon 140, 324 (2018).
K. Yang, Z.L. Yi, Q.F. **g, R.L. Yue, W. Jiang, and D.H. Lin, Sonication-assisted dispersion of carbon nanotubes in aqueous solutions of the anionic surfactant SDBS: the role of sonication energy. Chin. Sci. Bull. 58, 2082 (2013).
Y.M. Park, D.H. Lee, W.R. Hwang, S.B. Lee, and S.I. Jung, Hydrodynamics of CNT dispersion in high shear dispersion mixers. Korea Aust. Rheol. J. 26, 347 (2014).
S.C. Her and C.Y. Lai, Dynamic behavior of nanocomposites reinforced with multi-walled carbon nanotubes (MWCNT). Materials 6, 2274 (2013).
N. Sankar, N. Reddy, and R.K. Prasad, Carbon nanotubes dispersed polymer nanocomposites: mechanical, electrical, thermal properties and surface morphology. Bull. Mater. Sci. 39, 47 (2016).
V. Murudkar, A. Gaonkar, V.D. Deshpande, and S.T. Mhaske, Study of nano mechanical properties polydimethylsiloxane (PDMS)/MWCNT composites. AIP Conference Proceedings, vol. 1953 (American Institute of Physics Inc., 2018)
X. Zeng, X. Xu, P.M. Shenai, E. Kovalev, C. Baudot, N. Mathews, and Y. Zhao, Characteristics of the electrical percolation in carbon nanotubes/polymer nanocomposites. J. Phys. Chem. C 115, 21685 (2011).
H. Kim, E. Kim, C. Choi, and W.H. Yeo, Advances in soft and dry electrodes for wearable health monitoring devices. Micromachines (Basel) 13, 629 (2022).
J.D. Gibbons and S. Chakraborti, Nonparametric Statistical Inference (New York: Marcel Dekker, 2014).
Acknowledgments
This work is supported by the TIET-VT Center of Excellence for Emerging Materials (CEEMS). This Center of Excellence is jointly established by Thapar Institute of Engineering and Technology, Patiala, and Virginia Tech University (USA).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experimentation, data collection and analysis were performed by JP under supervision of co-authors. The first draft of the manuscript was written by JP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This research adhered to ethical standards concerning research involving both human and animal subjects. All necessary approvals were obtained from relevant ethical review boards and authorities, and all experiments were conducted in accordance with the approved protocols.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panchal, J., Singh, M.I., Sandha, K.S. et al. Rapid Fabrication Technique for Dry Electrocardiography Electrodes Using Carbon Nanotube/Polydimethylsiloxane Composite. J. Electron. Mater. 53, 2633–2645 (2024). https://doi.org/10.1007/s11664-024-10919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-10919-y